WEBINAIRE - REPLAY

Explore the journey of a chromatography project from lab to industrial

A step-by-step guide to de-risking
purification scale-up.
Each separation features unique characteristics. So, how can you derisk your purification process scale up? By developing the separation process through a checkpoint pathway.
This presentation will highlight a step-by-step approach used to ensure the development of a purification process, starting from laboratory throughout the whole life cycle of the industrial equipment.
An example of process development will be presented: the high fructose syrup (HFS) at 98% purity.
To watch this webinar replay, fill the form below and access immediately the online presentation.
Program
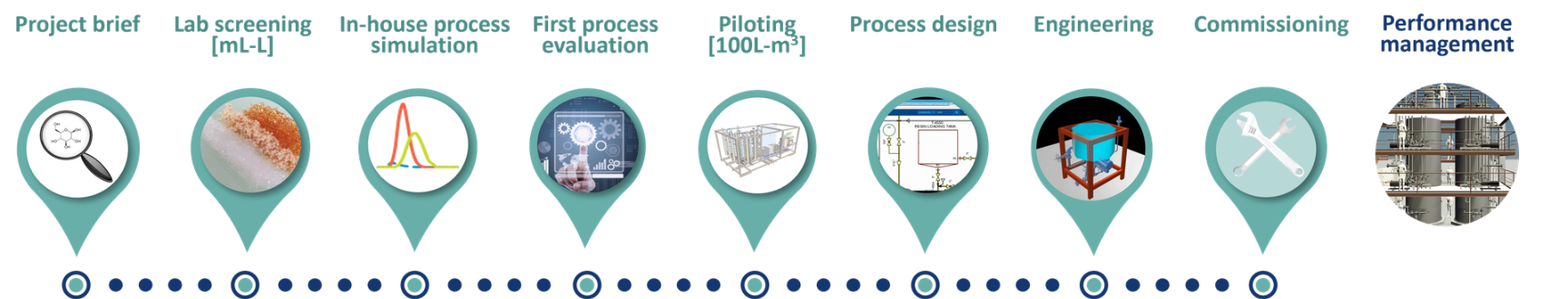
The step-by-step approach:
1. The project brief with the definition of feed material
2. The lab screening of the resin
3. The in-house numerical process simulation
4. The first process evaluation
5. The pilot scale validation
6. The process design
7. Engineering
8. Commissionning
It's for you if...
You want to:
- better control development time
- shorten time to market
- maximize the cost efficiency of the process
Speakers

Alexandra Gimbernat
PhD, Technology Product Manager
Alexandra Gimbernat joined Applexion in 2018 with a chemical engineering degree and a background in sugars through her PhD on sugar catalysis and separation process. She starts to work as part of the Research and Development team and now she works as Technology Product Manager focuses on digital services, a major part of Applexion New Tech initiative.

Hector Osuna-Sanchez
PhD, R&D Manager
Hector OSUNA is a PhD chemical engineer with expertise in purification processes for pharma, biopharma and industrial biotech. He joined Applexion in 2020 as R&D Manager, heading a 12-people team with lab and pilot capabilities coupled with numerical tools for efficient development and scale-up of purification industrial processes.
